What Is Meant by the Terms Unsaturated Saturated and Supersaturated
Unsaturated fats are typically liquid at room temperature. Answer 1 of 8.
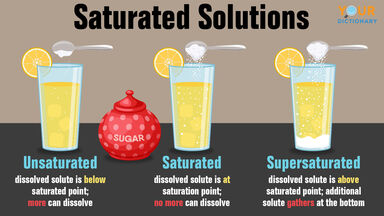
Supersaturated Solution Study Guide Inspirit
Determine the solubility in g solute100 g H2O.

. Monounsaturated fats are typically liquid at. A saturated solution of contains 180 g KCIO3 250 mL water at 200 degree C. A saturated solution is composed of the maximum amount of solutes that can be dissolved in a solvent at a given temperature.
View the full answer. Saturated and Unsaturated Solutions. Saturated solutions and supersaturated solutions are two such types.
A supersaturated solution holds more of the solvent than it would be able to. Is one in which more of the solute could dissolve at. A solution that contains more of the solute than what is present in its saturated solution at a particular temperature.
What is meant by the terms unsaturated saturated and supersaturated. A saturated solution is one that contains the maximum amount of solute capable of being dissolved whereas unsaturated solutions contain less than the maximum amount of solute capable of being dissolved. What is meant by the term solvated.
A solution is simply a mixture of a solute and a solvent. Why is the temperature at which crystals first form recorded instead of the temperature at which the crystals stop forming. The saturated solution can also be made unsaturated by adding more solvent to it.
A supersaturated solution contains more solute at a given temperature than is needed to form a saturated solution. When we speak of saturation with respect to solutions it is best to speak of an equilibrium condition In a saturated solution the solution holds an amount of solute that is equal to that amount that would be in equilibrium with UNDISSOLVED solute. Not all of the sugar crystals dissolved and a few settled on the bottom.
We review their content and use your feedback to keep the quality high. Unsaturated saturated and. An unsaturated solution contains less than the maximum soluble material while a saturated solution contains all of the material that it is able to dissolve in its current state with excess material remaining undissolved.
What is the difference between saturated unsaturated and supersaturated. Add second packet of sugar. What is meant when a solution is described as saturated unsaturated and supersaturated.
This type of unsaturated fat contains only one double bond in its structure. Saturated unsaturated and supersaturated refer to three different conditions of a solution. Previous question Next question.
They can be further categorized as. The most popular example is sodium acetate which is supersaturated. Is one in which no more of the solute will dissolve at a specific temperature.
A saturated solution contains the maximum. Solute particles are surrounded by solvent molecules. A supersaturated solution is a more solute solution than can be dissolved by the solvent.
Add one packet of sugar. The solubility at 50 C is 244 g100 mL of water. Increased temperature usually increases the solubility of solids in liquids.
Because carbonated water is saturated with carbon it emits carbon through bubbles. For example the solubility of glucose at 25 C is 91 g100 mL of water. A solution can be one of either gases or liquids.
A solvent is the gas or liquid in which the solute dissolves. There are three types of solutions. They differ from saturated fats in that their chemical structure contains one or more double bonds.
If you havent learned what a solute solvent is the material that is dissolved in the solution such as salts but not restricted to salts is a solution. Difference between Unsaturated Saturated and Supersaturated solutions. 100 2 ratings unsaturated solution Saturated solution supersaturated solution An unsaturated solution contains more of a sol.
The solution in which more solute can be added at given temperature is an unsaturated solution. A solute is matter that is dissolvable. Use models demonstrations and an instant hand warmer to help you teach this topicThis video is part of the Flinn Scientific Best Practices for Teaching Ch.
A supersaturated solution is composed of more than the amount of solutes that can be dissolved in a solvent at the same temperature. All of the sugar crystals dissolved with none settled on the bottom. Saturated solution-a solution that contains all the solute possible under equilibrium conditions at a given temperature.
Solubility vs Concentration - Basic Introduction Saturated Unsaturated and Supersaturated Solutions Saturated Definition and Example Saturated Unsaturated and supersaturated solution - video clip Types of solutions-saturated unsaturated supersaturated Tamil Saturated Solutions.

Difference Between Saturated And Unsaturated Solution Teachoo
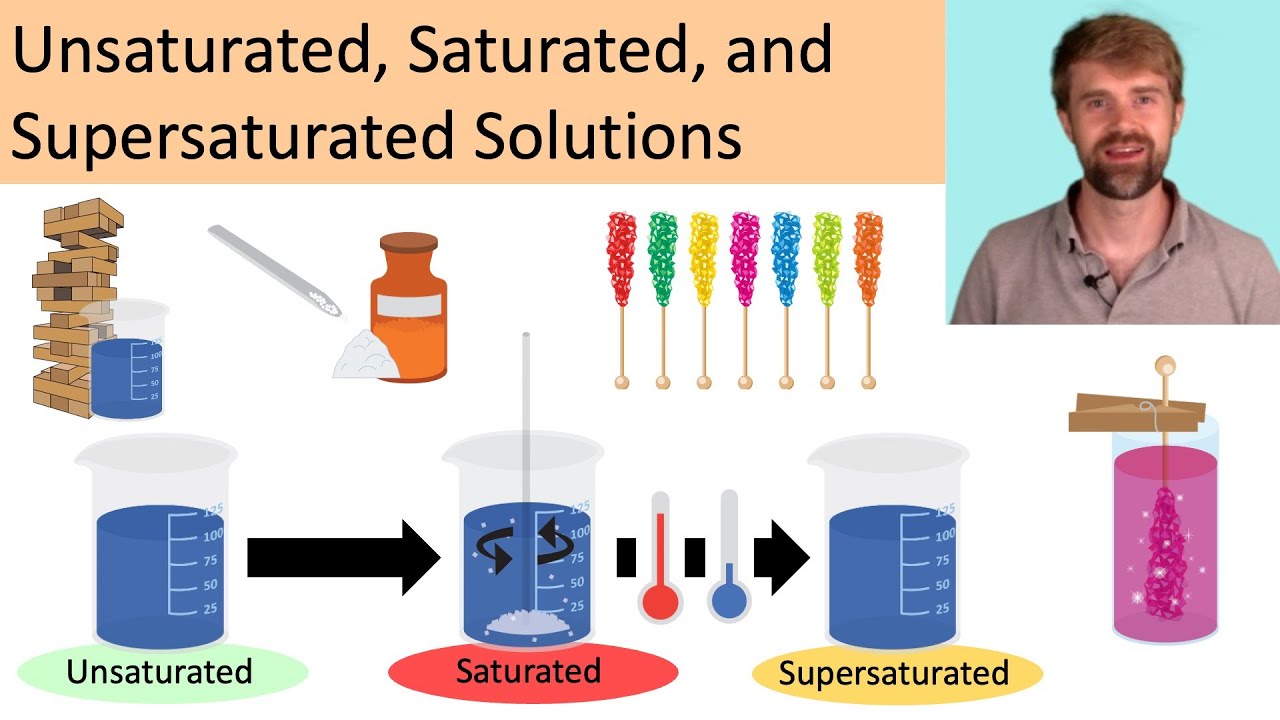
Unsaturated Saturated And Supersaturated Solutions Youtube
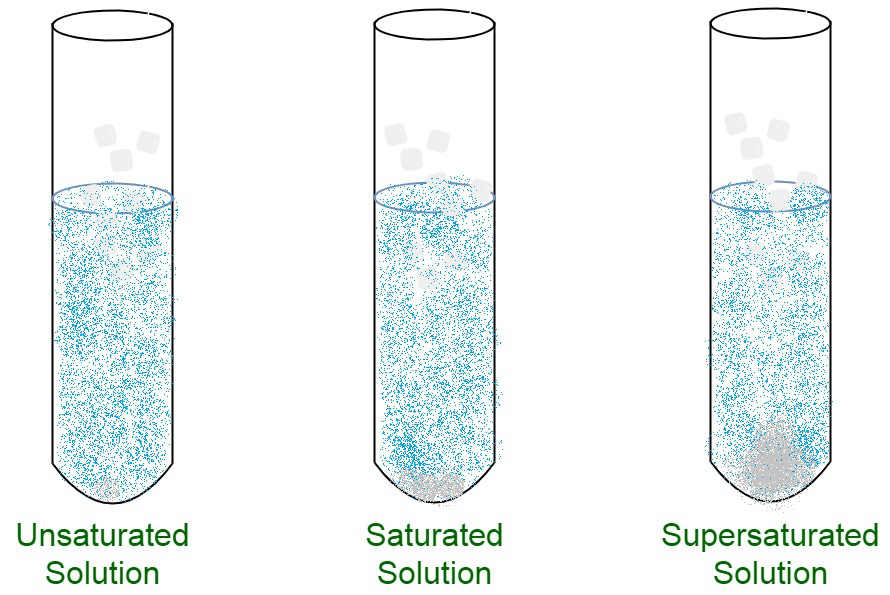
Saturated And Unsaturated Solutions Geeksforgeeks
How Are Saturated Unsaturated And Supersaturated Solutions Defined Quora
No comments for "What Is Meant by the Terms Unsaturated Saturated and Supersaturated"
Post a Comment